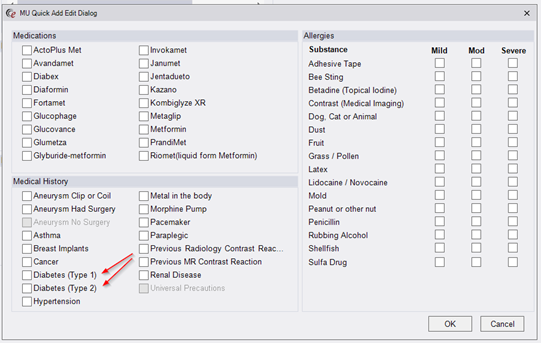
Previously, the MU Quick Add/Edit screen included an entry under Medical History for Diabetes, which was mapped to ICD10 code E11.9. However, it is preferable to provide two options for Diabetes to distinguish between Type 1 and Type 2. Therefore, the MU Quick Add Dialog has been modified to have Medical History options for both Type 1 and Type 2 diabetes.

These options are mapped to ICD10 codes E10 and E11.
This enhancement will allow all items that are still in Ordered status to be cancelled without affecting other items on the order that have already been scheduled. In the previous version of RIS, cancelling the order would be prevented because other studies on the order existed (and therefore the order must still exist). Now, choosing the Cancel Order action will allow the user to cancel the individual study while leaving studies in a more advanced status (along with the underlying order) intact. This is particularly helpful when one study on the order is canceled or discontinued and the Schedule Later option is chosen, putting the study back to Ordered status and displaying it on the Orders to Schedule WL. If the patient elects not to have the study, there will no longer be any difficulty removing it from the Orders to Schedule WL.
If the user right clicks and selects Cancel Order from the Orders to Schedule worklist or Patient Folder, the RIS will check for any other active studies on the order that have any status other than Cancelled. If one or more are detected, the following prompt will appear:
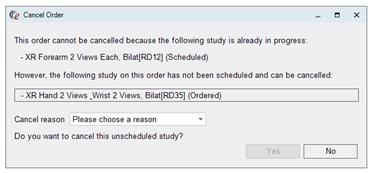
Note that the status of each of the studies is shown in parenthesis. If any studies on the order have previously been cancelled or discontinued, they will not be listed.
The user chooses a Cancel Reason and clicks Yes to continue with the cancellation of the study or studies that are not already in progress.
The following screenshot shows an order where two ordered studies are available for cancellation.
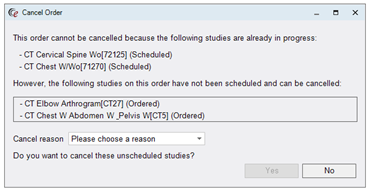
Furthermore, even if all of the studies on the order are still in Ordered status and can be cancelled, RIS will display a list of all studies to be sure the user intends to cancel the entire order. The Cancel Reason can be collected on the same pop-up screen and the user confirms their cancellation by clicking Yes.