![]()
Note that the Export Flag field is currently not used.
CURES
Summary
This enhancement delivers CURES update 170.315(b)(1), 170.315(b)(2)170.315(g)(6).
This enhancement for CURES updates RIS to support the additional data fields introduced by the adoption of the USCDI standard. In addition, the C-CDA creation, import, display, and export by RIS needs to include new required data fields introduced by the adoption of the USCDI standard.
This functionality was delivered via the Redmine tickets:
· Feature #29403 - CURES - 170.315(b)(1) C-CDA - Transitions of Care - USCDI Update
· Feature #29404 - CURES - 170.315(b)(2) C-CDA - Clinical Information Reconciliation and Incorporation - USCDI Update
· Feature #29407 - CURES a - 170.315(g)(6) C-CDA - Consolidated CDA Creation Performance - USCDI Update
Background
Previous CEHRT requirements required certain fields to always be included in the C-CDA, but an update to the USCDI standard has revised the set of data to be included.
Feature Description
With this change, both the C-CDA import and export from RIS have been updated to the new USCDI standard.
When performing a single-patient C-CDA export from the RIS UI, it can now export in both XML and HTML formats. Additionally, the new C-CDA may be saved locally, or transmitted via Direct Message.
Similarly, when exporting multiple patients/studies via the bulk C-CDA export (a back-end scheduled job), they can now be exported in both XML and HTML formats. The new C-CDAs are saved locally.
When performing a single-patient C-CDA import from the RIS UI via Direct Message, it can now import both the old and new C-CDA format.
Data Field Changes
The following RIS fields have been introduced to support the new USCDI standard:
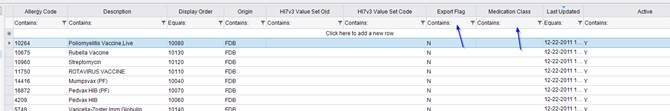
· The Allergy lookup table has been updated with new Medication Class and Export Flag fields.
|
|
Note that the Export Flag field is currently not used. |
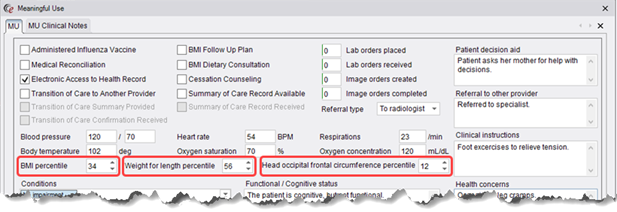
· The Meaningful Use dialog has been updated with new BMI percentile, Weight for length percentile, and Head occipital frontal circumference percentile fields required for new Vital Signs section entries in the C-CDA.
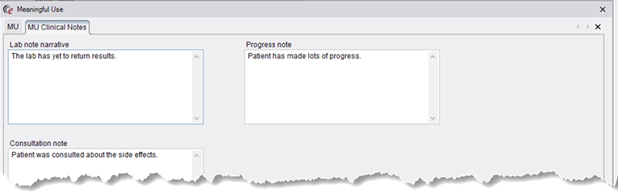
· The Meaningful Use dialog has a new MU Clinical Notes tab with new Lab note narrative, Progress note, and Consultation note fields.
C-CDA Generation
From a technical perspective, C-CDA documents were previously created by first turning the patient and study data into an XML document, then running that XML through an XSLT stylesheet to transform the data into a properly structured C-CDA document.
With this change, RIS now switches from using XSLT files to manipulate XML documents into a C-CDA, to now using a C-CDA generator library provided by Darena Solutions. This change is transparent to end users and C-CDAs will still be created, then either transmitted or exported to file by RIS just as before.
|
|
Service Team: Note that the Darena Solutions library update requires a .NET framework update to at least version 4.6.1, but this release increments it to version 4.8 for other features. |
Configuration Instructions
No System Administrator actions are necessary to enable this feature.
This enhancement delivers CURES update 170.315(e)(1) and 170.315(g)(9).
With this change, the C-CDA export from RIS has been updated to support the new fields introduced by adoption of the USCDI standard.
This functionality was delivered via the Redmine tickets:
· Feature #29405 - CURES - 170.315(e)(1) C-CDA - View, Download, and Transmit to a 3rd Party - USCDI Update
· Feature #29408 - CURES - 170.315(g)(9) C-CDA - Application Access - All Data Request - USCDI Update
Export via Patient Portal
With Feature #29405, the single-patient C-CDA export from the RIS Patient Portal was updated to export the updated C-CDA document in both XML and HTML formats.
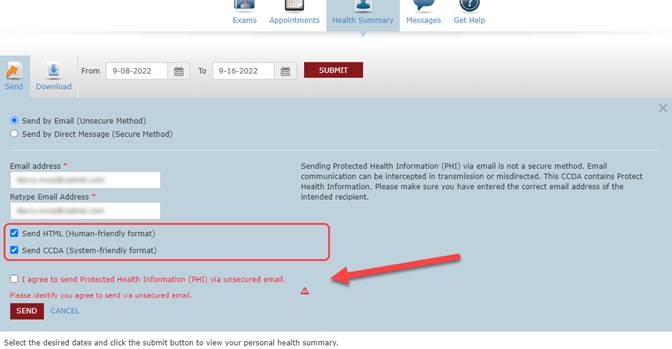
The confirmation check box must be selected before the email can be sent.
Exported files are attached to the email:
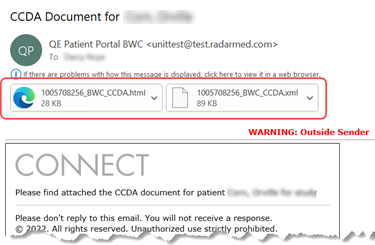
Export via API
With Feature #29408, the Patient Access API was also updated to support downloading the updated C-CDA in XML, HTML, or HTML4 formats.
Configuration Instructions
No System Administrator actions are necessary to enable this feature.
This enhancement delivers CURES update 170.315(D)(2) and CURES update 170.315(D)(3).
These two updates ensure that audit entries and report are still produced with the new USCDI elements.
This functionality was delivered via the Redmine tickets:
· Feature #29401 - CURES - 170.315(d)(3) Audit Report(s) - REVISED Criteria
· Feature #29400 - CURES - 170.315(d)(2) Adjustable Events and Tamper-Resistance - REVISED Criteria
With this change, the audit log will show entries for all exported and transmitted CCDAs with details on who exported and when, as well as a SHA-2 checksum.
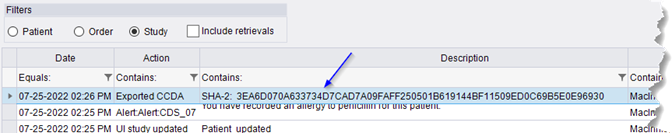
|
|
Note that the SHA-2 checksum is based on the XML, not the HTML version of the C-CDA. |
Configuration Instructions
No System Administrator actions are necessary to enable this feature.